Understanding your body’s electrolyte balance is crucial for overall health. The anion gap is a valuable tool healthcare professionals use to assess this balance and identify potential metabolic disturbances. This article provides a comprehensive guide to the anion gap, its calculation, interpretation, and clinical significance.
What is the Anion Gap?
Your blood contains a mix of positively charged particles (cations) and negatively charged particles (anions). The anion gap represents the difference between the concentrations of commonly measured cations (sodium, Na+) and anions (chloride, Cl-, and bicarbonate, HCO3-). This difference indirectly reflects the concentration of unmeasured anions in the blood.
Calculating the Anion Gap
The anion gap is calculated using the following formula:
Anion Gap (AG) = Na+ – (Cl- + HCO3-)
All values are measured in milliequivalents per liter (mEq/L). While a formula including potassium (K+) exists, it’s less commonly used in clinical practice.
Normal Anion Gap Range
The typical normal range for the anion gap is 8-12 mEq/L. However, this range can vary slightly between laboratories, so it’s always essential to refer to the specific reference range provided with your blood test results. Some labs use a range of up to 16 mEq/L.
Clinical Significance of the Anion Gap
The anion gap is primarily used to investigate metabolic acidosis, a condition where your blood becomes too acidic. An elevated anion gap suggests the presence of unmeasured anions contributing to this acidosis.
Elevated Anion Gap
An anion gap greater than 12-16 mEq/L often indicates metabolic acidosis. Several conditions can cause an elevated anion gap, including:
- Lactic Acidosis: A buildup of lactic acid, often due to inadequate oxygen supply to tissues. This may occur during intense exercise, shock, or severe infections.
- Diabetic Ketoacidosis (DKA): A serious complication of diabetes characterized by the accumulation of ketones in the blood.
- Kidney Failure: Impaired kidney function can lead to the retention of acidic waste products.
- Ingestion of Toxins: Certain toxins, such as methanol, ethylene glycol, and salicylates, can contribute to an elevated anion gap.
Decreased Anion Gap
While less common, a decreased anion gap (typically <8 mEq/L) can suggest other underlying issues, such as:
- Hypoalbuminemia: Low levels of albumin, a protein in your blood, can reduce the anion gap. Albumin is itself a negatively charged particle.
- Increased Cations: Elevated levels of cations like calcium (hypercalcemia) or magnesium (hypermagnesemia) can also lower the anion gap. Lithium toxicity can have a similar effect.
Normal Anion Gap Acidosis
It’s important to note that metabolic acidosis can occur even with a normal anion gap. This is often referred to as normal anion gap metabolic acidosis or hyperchloremic metabolic acidosis. Common causes include severe diarrhea and renal tubular acidosis. The urinary anion gap can help differentiate between gastrointestinal and renal causes of normal anion gap acidosis.
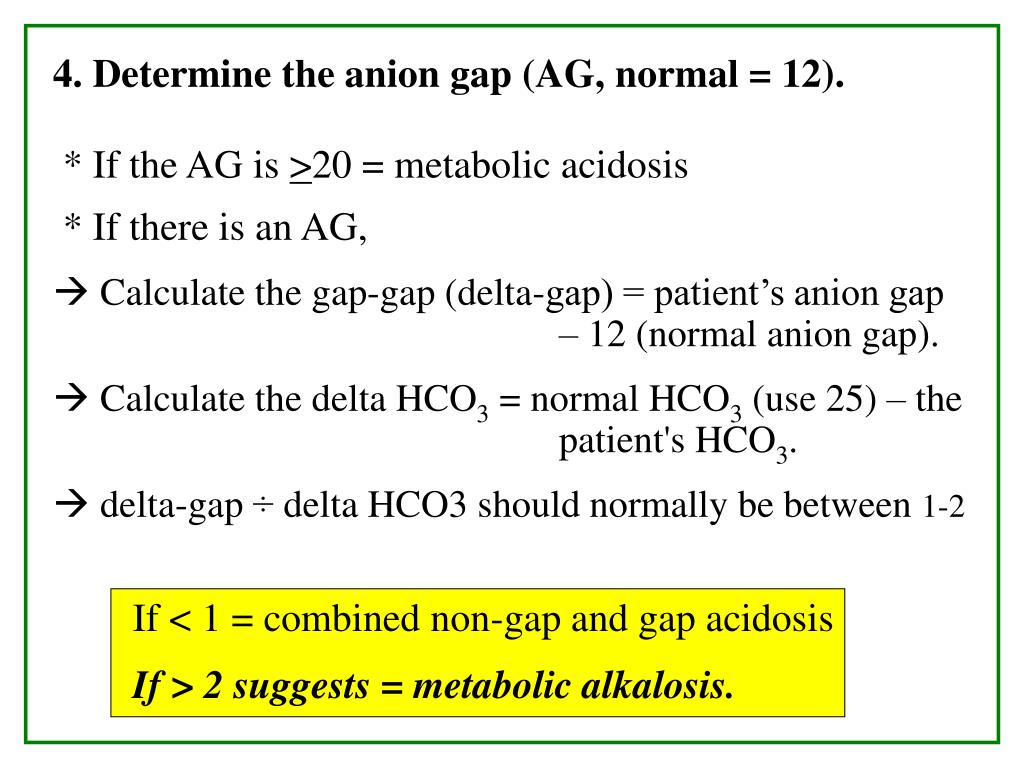
Advanced Metrics: Delta Gap and Delta Ratio
In more complex cases, clinicians may use additional calculations, such as the delta gap and delta ratio, to further refine their understanding of the acid-base disturbance. These metrics help assess mixed acid-base disorders and pinpoint the specific cause of metabolic acidosis.
Urinary Anion Gap: Clues from Your Kidneys
The urinary anion gap, calculated as (Na+ + K+) – Cl-, provides insights into the kidneys’ role in acid-base regulation. It can help differentiate between renal and gastrointestinal causes of normal anion gap acidosis. For instance, a positive urinary anion gap may suggest renal dysfunction, while a negative value could indicate gastrointestinal bicarbonate loss.
Anion Gap Calculators: A Convenient Tool
Several online anion gap calculators simplify the process of calculating the anion gap and other relevant metrics. These calculators typically require inputting serum sodium, chloride, and bicarbonate levels. Some calculators also provide the delta gap, delta ratio, and even offer interpretations based on the results.
Limitations of the Anion Gap
While the anion gap is a useful clinical tool, it’s crucial to acknowledge its limitations. Factors like lab-specific reference ranges, measurement errors, and certain medical conditions can influence the anion gap and its interpretation. Therefore, it’s essential to consider the anion gap in context with the patient’s overall clinical picture, including their medical history, physical exam findings, and other laboratory results.
How Doctors Use the Anion Gap
The anion gap is a valuable piece of the puzzle that healthcare professionals use to:
- Diagnose Metabolic Acidosis: Determine if someone has metabolic acidosis and consider the likely cause.
- Assess Severity: Gauge the significance of the electrolyte imbalance.
- Guide Treatment: Develop a targeted treatment plan addressing the underlying cause of the acidosis.
- Monitor Progress: Track a patient’s response to treatment and adjust as necessary.
Ongoing Research and Future Directions
Research continues to explore the anion gap and its role in various health conditions. Ongoing studies are investigating the most accurate reference ranges and optimal interpretation strategies for different patient populations, such as older adults or individuals with chronic kidney disease. There’s still more to learn about the full potential of the anion gap in understanding and managing electrolyte imbalances.
Conclusion
The anion gap is a powerful tool for evaluating acid-base balance and identifying potential metabolic disturbances. While the calculation is straightforward, understanding its interpretation and clinical implications is essential. Remember to consult with a healthcare professional for any health concerns or before making medical decisions. They can provide personalized guidance based on your individual circumstances. They can also assess if a toothache is contributing to a headache.
- Meal Prep Lunchbox Makes Healthy Eating Easy and Convenient - February 22, 2026
- Glass Rectangular Storage Containers Keep Your Kitchen Organized - February 21, 2026
- Stainless Containers Food Storage for Fresh, Organized Meals - February 20, 2026